TIBSOVO RESULTS: CHOLANGIOCARCINOMA (CCA) CLINICAL TRIAL
The TIBSOVO clinical trial was designed to determine if once-daily TIBSOVO could reduce the risk of disease progression or death in patients with advanced cholangiocarcinomaAdvanced cholangiocarcinoma means there are several tumors in the bile ducts. Metastatic cholangiocarcinoma means the cancer has spread to other places in the body, such as nearby tissue, lymph nodes, or more distant parts of the body. with an IDH1IDH1 stands for isocitrate dehydrogenase-1. In healthy cells, IDH1 plays an important role in normal chemical reactions. When IDH1 is altered due to a genetic mutation, it may promote the development of certain types of cancers, including cholangiocarcinoma. mutation whose disease had progressed after previous treatments.
TIBSOVO was studied in a clinical trial involving 185 adult patients with cholangiocarcinoma who:
- Had their cancer get worse after receiving at least 1 but not more than 2 previous treatment regimens
- Had disease that had spread
- Had a certain type of abnormal IDH1 mutation
The purpose of the trial was to determine how long patients lived without their cholangiocarcinoma getting worse.
TIBSOVO has been shown to increase the length of time a person can live with cholangiocarcinoma without it getting worse.
Benefits of TIBSOVO
- Patients treated with TIBSOVO achieved longer progression-free survivalProgression-free survival is the length of time during and after cancer treatment that a patient lives with the disease, but it does not get worse. In a clinical trial, measuring the progression-free survival is one way to see how well treatment works. Also called PFS. and better disease controlDisease control refers to the percentage of patients whose disease shrinks or remains stable over the study period in a clinical trial. compared with patients who received placebo
- Patients taking TIBSOVO had a lower risk of disease progression over 6 to 12 months
- More than half of the patients who took TIBSOVO achieved stable diseaseStable disease means neither an increase nor decrease in tumor size. vs 28% of patients who received placebo
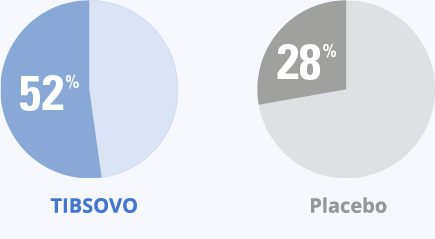
Patients taking TIBSOVO had higher rates of stable disease than those who took placebo.


